The science behind Lybrido
Previous attempts to develop a drug treatment for women with issues regarding desire or arousal have fallen into the “one-size-fits-all” approach, while failing to acknowledge the complexity of female sexuality.
Freya’s solutions are based on known neurobiological mechanisms that are important in sexual excitation and inhibition in women.
Lybrido focuses on treatment for patients with HSDD or FSIAD, in which low sexual desire results from a central nervous system that is relatively insensitive to sexual cues. In these individuals, exposure to sexual stimuli (internal or external) fails to trigger activation of the brain’s sexual excitatory mechanisms1-4.
Lybrido
Lybrido has been developed for patients with HSDD or FSIAD, who are relatively insensitive to sexual cues. It increases central sexual motivation and physiological sexual responses, such as swelling of vaginal erectile tissue and lubrication. Lybrido contains sildenafil (a phosphodiesterase type 5 inhibitor) with a time-release coating and an outer testosterone shell.
A Phase 3 trial for Lybrido is currently in preparation.
Three phase 2 studies (two for Lybridos and one for Lybrido) have been conducted at 16 research sites in the United States. The efficacy and safety of various doses of testosterone, sildenafil, buspirone and combination therapies (testosterone + sildenafil, testosterone + buspirone) were tested in 497 women with HSDD to determine the change in satisfying sexual events (SSEs) over a period of eight weeks.
In women with low sensitivity for sexual cues (Lybrido group), 0.5 mg testosterone + 50 mg sildenafil increased the number of SSEs by 170% compared with placebo, by 195% compared with sildenafil alone, and by 169% compared with testosterone alone.
In women with overactive inhibition (Lybridos group), 0.5 mg testosterone + 10 mg buspirone increased the number of SSEs by 99% compared with placebo, by 152% compared with buspirone alone, and by 98% compared with testosterone alone.
For further details on the clinical studies, see the Key Publication below.
The tablet is placed under the tongue for about 90 seconds, and then the remainder can be swallowed.
The reason for this two-way administration is so that the testosterone coating of the tablet can be absorbed directly into the bloodstream via the blood vessels under the tongue, while the tablet core dissolves slowly in the GI tract so that the sildenafil is released a few hours later.
Lybrido is designed to be taken only when desired – it is not necessary to take it daily.
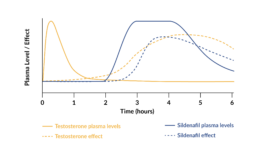
Sexual motivation is increased between 3 to 6 hours after intake.
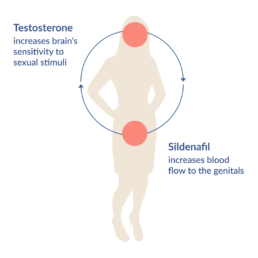
Lybrido is composed of a testosterone outer coating combined with a core of time-delayed sildenafil, which increases blood flow to the genitals.
Testosterone
Testosterone makes the brain more responsive to sexual cues, which in turn increases sexual motivation. A single dose of 0.5 mg testosterone under the tongue produces a brief increase in the blood plasma level of testosterone within 15 minutes, which returns to normal levels within 2 to 3 hours. During a period of 3 to 6 hours after peak plasma levels, there will be an increase in vaginal arousal and subjective sexual responses.
The peak in testosterone in Lybrido mimics the natural peak that occurs in women during ovulation, the phase of the menstrual cycle in which women typically experience increased sexual motivation and desire.
Sildenafil
The inner-core of Lybrido contains 50mg of sildenafil, which increases blood flow to the genitals. The increase in sexual motivation caused by the testosterone is required for its activation.
The sildenafil is coated with a time-delay substance to ensure that its release into the bloodstream occurs at the same time as the window of increased sexual motivation induced by the testosterone. This combination increases genital arousal by increasing responsiveness to sexual stimuli.
Tuiten A et al. Efficacy and safety of on-demand use of 2 treatments designed for different etiologies of Female Sexual Interest/Arousal Disorder: 3 randomized clinical trials. J Sex Med, 2018; 15: 201-216. https://www.sciencedirect.com/science/article/pii/S1743609517318519?via%3Dihub
2020
Gerritsen J et al. The effect of food on the pharmacokinetics of buspirone after single administration of a sublingual testosterone and oral buspirone combination tablet in healthy female subjects. Sex Med 2020 Jun; 8(2): 186-194. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7261678/
2019
Bloemers J et al.The effect of food on the pharmacokinetics of sildenafil after single administration of a sublingual testosterone and oral sildenafil combination tablet in healthy female subjects. J Sex Med 2019; 16: 1433-1443. https://academic.oup.com/jsm/article-abstract/16/9/1433/6966821?redirectedFrom=fulltext
2018
Tuiten A et al. Genotype scores predict drug efficacy in subtypes of female sexual interest/arousal disorder: A double-blind, randomized, placebo-controlled cross-over trial. Women’s Health, July 2018; 14. http://journals.sagepub.com/doi/full/10.1177/1745506518788970#articleShareContainer
Van Nes Y et al. Psychometric properties of the Sexual Event Diary in a sample of Dutch women with Female Sexual Interest /Arousal Disorder. J Sex Med, 2018; 15: 722-731. https://academic.oup.com/jsm/article-abstract/15/5/722/6980494?redirectedFrom=fulltext&login=false
2017
Van Nes Y et al. The Sexual Event Diary (SED): development and validation of a standardized questionnaire for assessing female sexual functioning over discrete sexual events. J Sex Med, 2017; 14: 1438-1450. https://academic.oup.com/jsm/article-abstract/14/11/1438/6973422?redirectedFrom=fulltext&login=false
2016
Bloemers J et al. Single dose sublingual testosterone and oral sildenafil versus a dual-route/dual-release fixed-dose combination tablet: a pharmacokinetic comparison. BJ Clin Pharm, 2016;81:1091-1102. https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.12887
2015
Suschinsky KD et al. The Clitoral Photoplethysmograph: A Pilot Study Examining Discriminant and Convergent Validity. Sex Med. 2015 Dec;12(12):2324-38. https://academic.oup.com/jsm/article-abstract/12/12/2324/6966780?redirectedFrom=fulltext&login=false
2014
van Rooij K et al. Efficacy of testosterone combined with a PDE5 inhibitor and testosterone combined with a serotonin 1A receptor agonist in women with SSRI-induced sexual dysfunction. Eur J Pharmacol, 2014. https://www.sciencedirect.com/science/article/abs/pii/S0014299914007973
van Rooij K et al. Pharmacokinetics of a prototype formulation of sublingual testosterone and a buspirone tablet, versus an advanced combination tablet of testosterone and buspirone in healthy premenopausal women. Drugs in R&D, 2014; 14: 125-32. https://link.springer.com/article/10.1007/s40268-014-0047-7
Poels S et al. Two novel combined drug treatments for women with Hypoactive Sexual Desire Disorder. Pharmacology, Biochemistry and Behavior, 2014; 121: 71-79. https://www.sciencedirect.com/science/article/abs/pii/S0091305714000318?via%3Dihub
2013
Bloemers J et al. Toward Personalized Sexual Medicine (Part 1): Integrating the “Dual Control Model” into Differential Drug Treatments for HSDD and FSAD. Journal of Sexual Medicine 2013; 10(3): 791-809. https://www.sciencedirect.com/science/article/abs/pii/S1743609515303088
Poels S et al. Toward Personalized Sexual Medicine (Part 2): Testosterone combined with a PDE5 inhibitor increases sexual satisfaction in women with HSDD and FSAD, and a low sensitive system for sexual cues. Journal of Sexual Medicine 2013;10 (3): 810-823. https://academic.oup.com/jsm/article-abstract/10/3/810/6939901?redirectedFrom=fulltext&login=false
van Rooij K et al. Toward Personalized Sexual Medicine (Part 3): Testosterone combined with a serotonin1A receptor agonist increases sexual satisfaction in women with HSDD and FSAD, and dysfunctional activation of sexual inhibitory mechanisms. Journal of Sexual Medicine 2013; 10(3): 824-837. https://academic.oup.com/jsm/article-abstract/10/3/824/6940114?redirectedFrom=fulltext&login=false
2012
van Rooij K et al. Pharmacokinetics of three doses of sublingual testosterone in healthy premenopausal women. Psychoneuroendocrinology 2012; 37(6): 773-81. https://www.sciencedirect.com/science/article/abs/pii/S0306453011002782?via%3Dihub
2009
van der Made F et al. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. Journal of Sexual Medicine 2009; 6(3): 777-90. https://academic.oup.com/jsm/article-abstract/6/3/777/6834448?redirectedFrom=fulltext&login=false
2002
Tuiten A et al. Can sublingual testosterone increase subjective and physiological measures of laboratory-induced sexual arousal? Archives of General Psychiatry, 2002; 59: 465–466. https://jamanetwork.com/journals/jamapsychiatry/article-abstract/206276
2000
Tuiten A et al. Time course of effects of testosterone administration on sexual arousal in women. Archives of General Psychiatry 2000; 57(2): 149-53. https://jamanetwork.com/journals/jamapsychiatry/fullarticle/481566

References
- Bloemers J, van Rooij K, Poels S, Goldstein I, Everaerd W, Koppeschaar H, Chivers M, Gerritsen J, van Ham D, Olivier B, Tuiten A. Toward personalized sexual medicine (part 1): integrating the “dual control model” into differential drug treatments for hypoactive sexual desire disorder and female sexual arousal disorder. J Sex Med 2013 Mar; 10(3): 791-809.
- van der Made F, Bloemers J, Yassem WE, Kleiverda G, Everaerd W, van Ham D, Olivier B, Koppeschaar H, Tuiten A. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med 2009 Mar; 6(3): 777-90.
- Poels S, Bloemers J, van Rooij K, Goldstein I, Gerritsen J, van Ham D, van Mameren F, Chivers M, Everaerd W, Koppeschaar H, Olivier B, Tuiten A. Toward personalized sexual medicine (part 2): testosterone combined with a PDE5 inhibitor increases sexual satisfaction in women with HSDD and FSAD, and a low sensitive system for sexual cues. J Sex Med 2013 Mar; 10(3): 810-23.
- van Rooij K, Poels S, Bloemers J, Goldstein I, Gerritsen J, van Ham D, van Mameren F, Chivers M, Everaerd W, Koppeschaar H, Olivier B, Tuiten A. Toward personalized sexual medicine (part 3): testosterone combined with a Serotonin1A receptor agonist increases sexual satisfaction in women with HSDD and FSAD, and dysfunctional activation of sexual inhibitory mechanisms. J Sex Med 2013 Mar; 10(3): 824-37.

References
- Bloemers J, van Rooij K, Poels S, Goldstein I, Everaerd W, Koppeschaar H, Chivers M, Gerritsen J, van Ham D, Olivier B, Tuiten A. Toward personalized sexual medicine (part 1): integrating the “dual control model” into differential drug treatments for hypoactive sexual desire disorder and female sexual arousal disorder. J Sex Med 2013 Mar; 10(3): 791-809.
- van der Made F, Bloemers J, Yassem WE, Kleiverda G, Everaerd W, van Ham D, Olivier B, Koppeschaar H, Tuiten A. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med 2009 Mar; 6(3): 777-90.
- Poels S, Bloemers J, van Rooij K, Goldstein I, Gerritsen J, van Ham D, van Mameren F, Chivers M, Everaerd W, Koppeschaar H, Olivier B, Tuiten A. Toward personalized sexual medicine (part 2): testosterone combined with a PDE5 inhibitor increases sexual satisfaction in women with HSDD and FSAD, and a low sensitive system for sexual cues. J Sex Med 2013 Mar; 10(3): 810-23.
- van Rooij K, Poels S, Bloemers J, Goldstein I, Gerritsen J, van Ham D, van Mameren F, Chivers M, Everaerd W, Koppeschaar H, Olivier B, Tuiten A. Toward personalized sexual medicine (part 3): testosterone combined with a Serotonin1A receptor agonist increases sexual satisfaction in women with HSDD and FSAD, and dysfunctional activation of sexual inhibitory mechanisms. J Sex Med 2013 Mar; 10(3): 824-37.
Freya Pharma Solutions B.V.
Kraanspoor 50 | 1033 SE Amsterdam | The Netherlands